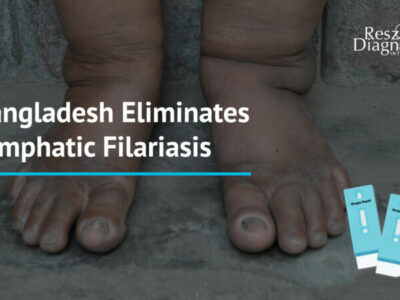
Circulating microfilariae can be detected by examining thick smears (20–60 μl) of finger-prick blood. Blood must be collected at a specific time – either at night or during the day – depending on the periodicity of the microfilariae. The method is inexpensive and feasible at individual and community levels for mapping the endemicity of lymphatic filariasis and monitoring mass drug administration (MDA).
The Brugia Rapid point-of-care cassette test manufactured by Reszon Diagnostics is recommended by WHO for use during Transmission Assessment Surveys (TAS) to detect IgG4 antibody against Brugia spp. in human blood samples.
Learn more about the product: bit.ly/2ALsxQp
Reference:
- World Health Organization. Diagnostic tests recommended for use in the Global Programme to Eliminate Lymphatic Filariasis. Who.int. Acessed on 10 January 2018.
- A Future Free of LF Alliance. Clinical Features, Diagnosis and Pathology. gaelf.com. Accessed on 10 January 2018.